 Antibodies against pathogens are necessary for the clearance of many infections, yet autoantibodies against self antigens drive the pathogenesis of
Antibodies against pathogens are necessary for the clearance of many infections, yet autoantibodies against self antigens drive the pathogenesis of
autoimmune disorders. In addition, humoral responses to certain pathogens can initiate autoimmunity in susceptible individuals. To better understand the regulation, interplay, and clinical effects of humoral immune responses, we study antibody production in infection, vaccination, and autoimmune disease.
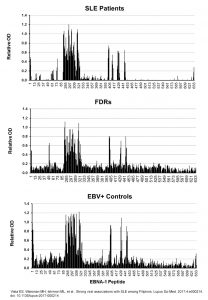
We have shown that Epstein-Barr virus (EBV) infection elicits distinct immune responses in people susceptible to lupus and multiple sclerosis, and that these responses can lead to pathogenic lupus autoimmunity through molecular mimicry and epitope spreading. Our ongoing studies examine the immune profiles, cellular phenotypes, and genetic factors that influence this process. We also use large, well characterized cohorts of lupus patients and high-risk individuals to understand the roles of EBV humoral immunity and viral reactivation in the onset of classified lupus.
We use anthrax vaccine adsorbed (AVA) as a model to understand what factors contribute to a protective or poor vaccine response. In collaboration with Walter Reed military sites, we established the largest real-world collection of samples from AVA-vaccinated individuals (>2,700 participants). Less than 50% of these vaccinees showed protective responses in an in vitro lethal toxin neutralization assay. Our ongoing studies examine how antibody chemistry, B cell activation, and antigen presentation influence the development of poor vaccine responses after anthrax vaccination.
Together, these studies provide basic insights to the nature of human humoral responses as well as a foundation for future rational design of immunotherapeutics.
Browse other areas:
Pre-clinical Autoimmunity
Mechanisms of Disease Flare
Deciphering Molecular Heterogeneity
Improving Health Equity